Publications
Wenceslau, et al.
Hypertension — February 2024
To celebrate 100 years of AHA-supported cardiovascular disease research, this review article highlights milestone papers that have significantly contributed to the current understanding of the signaling mechanisms driving hypertension and associated cardiovascular disorders. This article also includes a few of the future research directions arising from these critical findings. To accomplish this important mission, 4 principal investigators gathered their efforts to cover distinct yet intricately related areas of signaling mechanisms pertaining to the pathogenesis of hypertension. The renin-angiotensin system, canonical and novel contractile and vasodilatory pathways in the resistance vasculature, vascular smooth muscle regulation by membrane channels, and noncanonical regulation of blood pressure and vascular function will be described and discussed as major subjects.
Costa, McCarthy, et al.
Clinical Science — November 2023
O-Linked attachment of β-N-acetylglucosamine (O-GlcNAc) on serine and threonine residues of nuclear, cytoplasmic, and mitochondrial proteins is a highly dynamic and ubiquitous post-translational modification that impacts the function, activity, subcellular localization, and stability of target proteins. Physiologically, acute O-GlcNAcylation serves primarily to modulate cellular signaling and transcription regulatory pathways in response to nutrients and stress. To date, thousands of proteins have been revealed to be O-GlcNAcylated and this number continues to grow as the technology for the detection of O-GlcNAc improves. The attachment of a single O-GlcNAc is catalyzed by the enzyme O-GlcNAc transferase (OGT), and their removal is catalyzed by O-GlcNAcase (OGA). O-GlcNAcylation is regulated by the metabolism of glucose via the hexosamine biosynthesis pathway, and the metabolic abnormalities associated with pathophysiological conditions are all associated with increased flux through this pathway and elevate O-GlcNAc levels. While chronic O-GlcNAcylation is well associated with cardiovascular dysfunction, only until recently, and with genetically modified animals, has O-GlcNAcylation as a contributing mechanism of cardiovascular disease emerged. This review will address and critically evaluate the current literature on the role of O-GlcNAcylation in vascular physiology, with a view that this pathway can offer novel targets for the treatment and prevention of cardiovascular diseases.
Pernomian, Wenceslau, et al.
ELSEVIER — October 2023
The major pathophysiological characteristic of hypertension is the occurrence of small artery remodeling and endothelial dysfunction. There is also solid evidence showing that microcirculation abnormalities occur prior to the onset of hypertension. However, the mechanism(s) that trigger these changes prior to the elevation of blood pressure are unknown, and this may limit our ability to identify the cause of this disease and effectively treat it. In hypertension, as with aging, the vasculature becomes less susceptible to repair. One of the reasons is because endothelial cells start to deteriorate and present with exacerbated endothelial-to-mesenchymal transition (EndMT). Likewise, vascular smooth muscle cells (VSMC) also dedifferentiate into a synthetic phenotype, whereby they start to produce and secrete extracellular vesicles with a high migration and proliferation capacity for repairing vascular injury. Uncontrolled EndMT and/or VSMC phenotype switching contributes to vascular diseases, but the initial trigger for these conditions is unidentified. Importantly, EndMT and synthetic VSMC exhibit plasticity and can return to adopt an endothelial cell-like fate and present contractile phenotype again, respectively. Therefore, in this hypothesis we will take advantage of this plasticity, and we propose to manipulate this fate by inducing partial cellular reprogramming without passing through the pluripotent state. Specifically, we suggest that activation of the three master transcription factors, Oct-4, Sox-2, and Klf-4 (collectively termed OSK) will reprogram endothelial cells and prevent and reduce EndMT and VSMC synthetic phenotype. It was recently shown that activation of OSK was able to restore lost vision in old mice, and cancer risk was reduced by excluding c-Myc. Therefore, OSK treatment could provide new possibilities for vascular rejuvenation and treatment of hypertension.
Waigi, Wenceslau, et al.
GeroScience — June 2023
Dementia refers to a particular group of symptoms characterized by difficulties with memory, language, problem-solving, and other thinking skills that affect a person’s ability to perform everyday activities. Alzheimer’s disease (AD) is the most common form of dementia, affecting about 6.2 million Americans aged 65 years and older. Likewise, cardiovascular diseases (CVDs) are a major cause of disability and premature death, impacting 126.9 million adults in the USA, a number that increases with age. Consequently, CVDs and cardiovascular risk factors are associated with an increased risk of AD and cognitive impairment. They share important age-related cardiometabolic and lifestyle risk factors, that make them among the leading causes of death. Additionally, there are several premises and hypotheses about the mechanisms underlying the association between AD and CVD. Although AD and CVD may be considered deleterious to health, the study of their combination constitutes a clinical challenge, and investigations to understand the mechanistic pathways for the cause-effect and/or shared pathology between these two disease constellations remains an active area of research. AD pathology is propagated by the amyloid β (Aβ) peptides. These peptides give rise to small, toxic, and soluble Aβ oligomers (SPOs) that are nonfibrillar, and it is their levels that show a robust correlation with the extent of cognitive impairment. This review will elucidate the interplay between the effects of accumulating SPOs in AD and CVDs, the resulting ER stress response, and their role in vascular dysfunction. We will also address the potential underlying mechanisms, including the possibility that SPOs are among the causes of vascular injury in CVD associated with cognitive decline. By revealing common mechanistic underpinnings of AD and CVD, we hope that novel experimental therapeutics can be designed to reduce the burden of these devastating diseases.
Wenceslau, McCarthy, et al.
American Journal of Physiology : Heart and Circulatory — June 2021
The measurement of vascular function in isolated vessels has revealed important insights into the structural, functional, and biomechanical features of the normal and diseased cardiovascular system and has provided a molecular understanding of the cells that constitutes arteries and veins and their interaction. Further, this approach has allowed the discovery of vital pharmacological treatments for cardiovascular diseases. However, the expansion of the vascular physiology field has also brought new concerns over scientific rigor and reproducibility. Therefore, it is appropriate to set guidelines for the best practices of evaluating vascular function in isolated vessels. These guidelines are a comprehensive document detailing the best practices and pitfalls for the assessment of function in large and small arteries and veins. Herein, we bring together experts in the field of vascular physiology with the purpose of developing guidelines for evaluating ex vivo vascular function. By using this document, vascular physiologists will have consistency among methodological approaches, producing more reliable and reproducible results.
Edwards, Roy, et al.
Hypertension — March 2021
Cell death has long been a characteristic phenotype of organ damage in hypertension, and recently, leaky gut has been revealed as a novel hypertensive phenotype. However, despite the increase in bacterial and damaged mitochondrial products in the circulation of hypertensive patients and animals, the mechanistic contribution of these two phenomena to hypertension pathophysiology is unknown. Mitochondria and bacteria both start protein translation with an N-formyl methionine residue and thus are the only sources of NFPs (N-formyl peptides), which activate the FPR-1 (formyl peptide receptor-1). We hypothesized that the synergistic action of bacterial and mitochondrial NFPs would cause the spontaneous elevation of blood pressure and vascular remodeling in male Dahl salt-sensitive rats via FPR-1. We observed that mitochondria-derived peptides originating from cell death in the kidneys are responsible for FPR-1–induced vascular hypercontractility and remodeling and premature elevation of BP in Dahl salt-sensitive rats fed a low-salt diet. However, a high-salt diet leads to gut barrier disruption and, subsequently, a synergistic action of mitochondria, and bacteria-derived leaky gut NFPs lead to a severe and established hypertension. Administration of an FPR-1 antagonist lowered blood pressure in Dahl salt-sensitive rats on a low-salt diet but amoxicillin administration did not. These results reveal for the first time that cell death can be a cause of hypertensive pathophysiology, whereas leaky gut is a consequence.
Roy, Edwards, et al
Function (Oxf) — November 2020
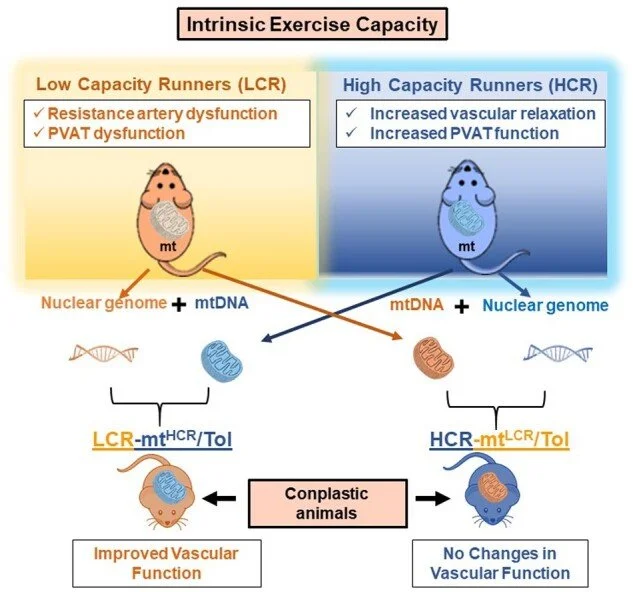
Exercise capacity is a strong predictor of all-cause morbidity and mortality in humans. However, the associated hemodynamic traits that link this valuable indicator to its subsequent disease risks are numerable. Additionally, exercise capacity has a substantial heritable component and genome-wide screening indicates a vast amount of nuclear and mitochondrial DNA (mtDNA) markers are significantly associated with traits of physical performance. A long-term selection experiment in rats confirms a divide for cardiovascular risks between low- and high-capacity runners (LCR and HCR, respectively), equipping us with a preclinical animal model to uncover new mechanisms. Here, we evaluated the LCR and HCR rat model system for differences in vascular function at the arterial resistance level. Consistent with the known divide between health and disease, we observed that LCR rats present with resistance artery and perivascular adipose tissue dysfunction compared to HCR rats that mimic qualities important for health, including improved vascular relaxation. Uniquely, we show by generating conplastic strains, which LCR males with mtDNA of female HCR (LCR-mtHCR/Tol) present with improved vascular function. Conversely, HCR-mtLCR/Tol rats displayed indices for cardiac dysfunction. The outcome of this study suggests that the interplay between the nuclear genome and the maternally inherited mitochondrial genome with high intrinsic exercise capacity is a significant factor for improved vascular physiology, and animal models developed on an interaction between nuclear and mtDNA are valuable new tools for probing vascular risk factors in the offspring.
Additional Publications
McCarthy CG, Waigi EW, Yeoh BS, Mell B, Vijay-Kumar M, Wenceslau CF, Joe B. Low-dose 1,3-butanediol reverses age-associated vascular dysfunction independent of ketone body β-hydroxybutyrate. Am J Physiol Heart Circ Physiol. 2022 Mar 1;322(3):H466-H473. doi: 10.1152/ajpheart.00486.2021. Epub 2022 Feb 11. PubMed PMID: 35148235; PubMed Central PMCID: PMC8897007.
Costa, Tiago J.a,b; Linder, Braxton A.c; Hester, Setha,b; Fontes, Milenea,b; Pernomian, Laenaa,b; Wenceslau, Camilla F.a,b; Robinson, Austin T.c; McCarthy, Cameron G.a,b. The janus face of ketone bodies in hypertension. Journal of Hypertension 40(11):p 2111-2119, November 2022. | DOI: 10.1097/HJH.0000000000003243
Santos CVD, Kerkhoff J, Tomazelli CA, Wenceslau CF, Sinhorin AP, de Jesus Rodrigues D, Carneiro FS, Bomfim GF. Vasoconstrictor and hemodynamic effects of a methanolic extract from Rhinella marina toad poison. Toxicon. 2022 Oct 30;218:57-65. doi: 10.1016/j.toxicon.2022.08.018. Epub 2022 Sep 14. PMID: 36113683; PMCID: PMC9832923.
McCarthy CG, Waigi EW, Singh G, Castaneda TR, Mell B, Chakraborty S, Wenceslau CF, Joe B. Physiologic, Metabolic, and Toxicologic Profile of 1,3-Butanediol. J Pharmacol Exp Ther. 2021 Nov;379(3):245-252. doi: 10.1124/jpet.121.000796. Epub 2021 Sep 14. PubMed PMID: 34521698; PubMed Central PMCID: PMC9164310.
McCarthy CG, Chakraborty S, Singh G, Yeoh BS, Schreckenberger ZJ, Singh A, Mell B, Bearss NR, Yang T, Cheng X, Vijay-Kumar M, Wenceslau CF, Joe B. Ketone body β-hydroxybutyrate is an autophagy-dependent vasodilator. JCI Insight. 2021 Oct 22;6(20). doi: 10.1172/jci.insight.149037. PubMed PMID: 34499623; PubMed Central PMCID: PMC8564907.
McCarthy CG, Saha P, Golonka RM, Wenceslau CF, Joe B, Vijay-Kumar M. Innate Immune Cells and Hypertension: Neutrophils and Neutrophil Extracellular Traps (NETs). Compr Physiol. 2021 Feb 12;11(1):1575-1589. doi: 10.1002/cphy.c200020. Review. PubMed PMID: 33577121; PubMed Central PMCID: PMC9721119.
Schreckenberger ZJ, Wenceslau CF, Joe B, McCarthy CG. Mitophagy in Hypertension-Associated Premature Vascular Aging. Am J Hypertens. 2020 Sep 10;33(9):804-812. doi: 10.1093/ajh/hpaa058. Review. PubMed PMID: 32533696; PubMed Central PMCID: PMC7481986.
McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC. Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2019 Nov 1;317(5):H1013-H1027. doi: 10.1152/ajpheart.00227.2019. Epub 2019 Aug 30. PubMed PMID: 31469290; PubMed Central PMCID: PMC6879927.
Cheon S, Tomcho JC, Edwards JM, Bearss NR, Waigi E, Joe B, McCarthy CG, Wenceslau CF. Opioids Cause Sex-Specific Vascular Changes via Cofilin-Extracellular Signal-Regulated Kinase Signaling: Female Mice Present Higher Risk of Developing Morphine-Induced Vascular Dysfunction than Male Mice. J Vasc Res. 2021;58(6):392-402. doi: 10.1159/000517555. Epub 2021 Sep 14. PMID: 34521095; PMCID: PMC8612963.
Silva CBP, Elias-Oliveira J, McCarthy CG, Wenceslau CF, Carlos D, Tostes RC. Ethanol: striking the cardiovascular system by harming the gut microbiota. Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H275-H291. doi: 10.1152/ajpheart.00225.2021. Epub 2021 Jun 18. PMID: 34142885; PMCID: PMC8410123.